Student researchers in the annual Research Experiences for Undergraduates (REU) program use trees as model systems to investigate questions related to evolution, ecology, conservation, and management in natural and built environments.
Individuals gain direct experience in all aspects of a research project, from researching the primary literature to sampling design, collecting and analyzing data, and presenting at a final symposium.
Learn about the projects and experiences of the 2024 student researchers in this Student Blog Series.
Hank Helmers
Summer 2025, Rose-Hulman Institute of Technology
Tree Conservation Biology Lab
“Sticky Fingers and Silver Rivers: A Summer with the Butternuts”
Our fingertips lay sticky with reds and purples. The kids outstretched their tiny fingers, displaying them with a giggling alarm. I need a bandage! They would say. But we all knew better, for we too stood arms deep in the black raspberry bushes. In an opening of a deciduous forest where the berries are particularly abundant, no one was safe from their draw. Everyone, from the retired forest pathologist in our group to the youngest member of the indigenous youth education program visiting that day, was giddy with the generosity of the forest. The lot of us painted a glittering image of enjoyment. However, we were not just there on a berry expedition; rather, we had a mission.
Just due north of the berry clearing stood a striking tree (see above image). This tree was our mission. This was a butternut, or white walnut, tree (Juglans cinerea). The tree was tall with silver bark carving smooth rivers through the trunk. Taking her all in, the branches stood apart as they stretched to the sky fruitlessly. Stepping a bit closer, we all notice the trunk covered in deep black cracks. These black cracks, or cankers, are indicative of butternut canker disease, and over the last 60 years, this disease has caused a decline of 90% of all butternut populations across the Eastern US. Consequently, butternuts are in great need of conservation. Unfortunately, it appears this tree hasn’t escaped the disease. Since the tree lacked any leaves, we concluded it was dead (see image below).
Yet, not all trees are affected the same. Just a few steps away, another butternut stood in its full glory (see image above). Backlit by a crystal-clear sky, the team stood in awe of what seemed to be a rare sight: a healthy butternut. As we made our way through the field site, more and more inconsistencies in butternut health appeared. Despite over 90% of the nationwide population dying from this disease, many of the trees we found in this location still had most of their leaves, meaning they were still able to obtain energy, grow, and possibly flower. Some even had seeds! Sure, none had appeared to escape the blackened cracks of the disease, but it was clear that not all trees are affected equally. So, what gives? Why are some trees appearing healthy and others dying off? These are the very questions I explored during my summer in the Research Experiences for Undergraduates (REU) program at The Morton Arboretum.
Hi, my name is Hank Helmers, a computer science and biology double major at Rose-Hulman Institute of Technology. As part of the Tree Conservation Biology Program, I had the pleasure of being mentored by Dr. Sean Hoban, the Senior Scientist of the Tree Conservation Biology program, and Emma Leavens, the Program Manager. Both of these scientists have graciously and passionately introduced me to the world of butternuts and their projects aimed at conserving this illustrious species. I got to join the project focused on butternut health. Specifically, my project was to investigate the health of these existing butternut populations and the environments in which they live.
This project began by developing a clear understanding of how to assess butternut health through the creation of a health assessment form. We identified the essential observations for signs of health and stress on the tree, such as the percentage of live leaves, seed production, and the percentage of the trunk affected by canker. Next, we added environmental factors that could impact health to the form, such as shading, nearby tree species, and competition.
Notably, more than one environment of butternut populations was included in our observations of butternut health. As a part of this project, I had the fortunate opportunity to explore butternuts in the Chicago region and in Vermont. Studying a variety of populations allows us to identify differences in how butternuts are doing in different parts of the country. Thus, to understand how different environments affect butternuts’ health, selecting diverse site locations is essential.
With a consistent form of health questions arranged and locations identified, we could make our way to the field! Where, alongside berry-picking and joyous baby butternut findings, we collectively estimated the described observations. All of these field expeditions culminated in a lot of data. So, data management and analysis rounded out my summer, allowing me to utilize my computer skills and begin answering our questions!
Although my statistical analysis is still pending, we observed a lot of new information about butternut health. Most notably, there are tons of new butternut seedlings (see above image). Our Vermont site alone had over 600+ seedlings ranging from a few weeks to a few years old. Why are there so many new seedlings? Have these seedlings just now started sprouting? Or have they gone unnoticed and died off? Despite all of the unknowns, the presence of all these seedlings inspires hope for the possibility of butternut regeneration! It was once thought that butternuts were not producing new seedlings or that seedlings could not survive the first few years. But, as we’ve seen this year, this is not true. If new seedlings can sprout and survive to adulthood, eventually producing their own seeds, then butternuts are functionally recovering!
As such, it is more important than ever to monitor and study these seedlings from year to year. By exploring butternut health across multiple environments and years, we can make connections between what healthy individuals look like and identify where they are doing well! With this information, we can inform conservation efforts, such as knowing which butternuts to protect and where to plant new ones. In this way, the information research provides is necessary for conservation. But what I’ve begun to appreciate this summer is that conservation isn’t only about research.
Yes, research gives us new information about butternuts. How healthy are they? Where are they healthy? And why? Okay, great. Suppose we do identify the right conditions for butternut. Time to plant new ones! However, where are we going to find seed? And how are we going to get it? Then, who will do this planting and ensure it’s done well? This is where collaboration with indigenous communities, foresters, land stewards, and public conservationists is paramount.
Historically, butternut has been of great cultural importance to indigenous communities. In particular, the namesake butternuts served as a versatile resource for food, dyes, and oils. Consequently, butternut trees are an important piece of indigenous cultural history, meaning that the context of indigenous knowledge is an essential piece of butternut conservation knowledge. Other valuable collaborations include foresters who study and manage public forests, as well as land stewards who encompass communities that manage and care for other land resources, from forest preserves to school yards.
All of these collaborators are focused on the long-term sustainability and conservation of their lands. Their knowledge and experience with the lands themselves are vital information to conservation, which science alone cannot provide. Therefore, to ensure butternut health and regeneration, these collaborations are necessary to holistically and realistically conserve butternuts. Butternuts on these lands can then be protected and planted as informed by all parties. That is, if all parties are invested and knowledgeable about the state of butternuts and the steps they can take for butternut restoration.
To ensure the public is knowledgeable and has access to new, high-quality butternut seeds, we rely on public conservationist organizations like the Forest Gene Conservation Association (FGCA) in Canada. They also study butternuts and were with us in the field to share observations and insights from their own butternut monitoring work. They also educate and inspire butternut conservation among general audiences. With the public engaged, they utilize their seed orchards to provide their province with genetically diverse sources of butternut seeds and guide collaborators on how to best care for butternuts. And how do we know how to best care for butternuts: research.
Clearly, science and research alone are not enough to encompass a holistic view of the cultural and natural histories of butternuts. Instead, a diverse collaboration is needed to ensure the sustenance and renewal of this species. This diversity of perspectives and knowledge from indigenous knowledge, forestry and restoration stewardship, conservation research, and local engagement highlights the deeply collaborative and communal foundation underpinning efforts to conserve a species or ecological community.
Alongside our field expeditions, I had the pleasure of meeting individuals from across these perspectives, all of which have helped me to solidify and inspire my hope in conservation work. Notably, from all these perspectives, notice the diversity of work needed to do conservation. Just as an ecosystem relies on diversity to remain resilient and sustain health, conservation relies on a diversity of people contributing their expertise and passions.
Being surrounded by this variety of conservationists, I have been reminded of the beauty and fun of nature. While field days served an important scientific purpose, everyone was excited about the future of butternuts. Our work often felt like a childhood walk in the woods, following the trails of our curiosity as much as anything. A proud Kentuckian, I gravitated to the wild from the start. Not a summer day went by that my sisters and I couldn’t be found barefoot and curious in the muddy hills, bothering snakes and swinging from hackberry trees. And by some stroke of luck, I find myself here with that same joy.
This joyful work has exposed me to a concrete concept of how the conservation of a species can look. It’s not just looking at trees, it’s not just ecology, it’s not just forestry, it’s not even just berries. It’s everything. It takes everyone, from scientists to communities, to choose hope and resilience in their ability to engage with nature. And if anything symbolized resilience, it is the butternut tree. She continues to confound us in her persevering spirit, and we still have a lot to learn about her and what it will take to conserve her. For once, however, I know this team can get there. One berry-full day at a time.
Cierra Lizer
Summer 2025, St. Olaf College
Forest Ecology Lab
“Patterns of Autumn Leaf Phenology Following Drought”
The Morton Arboretum is well known for the sea of color it puts on in the fall, rich hues of oranges, reds, and golds that are a wonder to see. But, beyond the beauty, this time of year is incredibly important for how forests stay healthy and recycle their valuable nutrients.
As a summer intern eager to explore the impacts of climate change on trees, I came onto the Morton Arboretum Forest Ecology lab’s long term project, perfect for this goal. For years, members of the lab have collected leaf litter from the Arboretum’s East Woods using our leaf litter traps, that are just tomato cages with mesh draped over to catch the falling debris. How much leaves fell, when they fell, and what’s inside the leaves can clue us into some really important processes at work. I am lucky enough to have come onto this project to not only continue collecting litter but I also get to be the first to look at this massive treasure trove of data.
There is still a lack of understanding about what factors contribute to when and why trees drop their leaves during autumn. I was quite interested in how stress, like drought, impact this timing of leaf drop. There seems to be ideas for both sides: drought makes leaves fall earlier sometimes, but also makes leaves fall later others. Therefore, my project is about digging into these leaf samples and our extensive weather records.
The first step of this is to sort through all of the litter we have collected. Imagine bags and bags of these dried specimens. Me and many others from my lab sort flower from fruit from leaf of each species. It’s oddly satisfying to go from a dry heap of organic matter to plates of organized specimens.
Not only do I want to know if there is a change in the timing of leaf fall during drought, but I also want to understand why it might do this. To accomplish this, I am also looking at what’s inside these leaves, specifically, how much nitrogen there is. Nitrogen is a key nutrient for trees, and we think if a tree drops its leaves really fast because it’s stressed by a drought, it might not have time to pull all its valuable nutrients back inside. So, more nitrogen left in the fallen leaf could be a sign the tree was in a hurry due to stress, and less nitrogen might mean the opposite. To investigate the nitrogen, I grind up the leaves into really small pieces, put them in tiny foil tins, and run them through a machine that will tell me how much nitrogen is in the sample.
While this is a cool and fun science puzzle, it could also help us predict how the Morton Arboretum’s forests, and other forests around the world, will handle a changing climate and frequent or more intense droughts in the future.
Life at the Arboretum isn’t just about leaves, though! The REU interns have gotten really close and regularly plan activities around the Arboretum and into Chicago. Sometimes, before the workday even begins, some of us researchers sneak in a morning birding session. Just last week, we spotted a majestic blue heron by the lake, a perfect reminder of the vibrant life we’re working to understand and protect.
Edlyn Martinez
Summer 2025, Harold Washington College
Soil Ecology Lab
“Brush pile burn restoration techniques”
I love puzzles.
Coming from a community college, knowing what path your major is going to take you isn’t always very clear. I was taking my general education courses, a few classes that touched on environmental science, but nothing that helped me see the bigger picture. I hadn’t had access to fieldwork or lab experience either, which are two crucial components of environmental science. Because what I was learning was so broad, as well as my lack of experience, I didn’t know what kind of work I’d be able to do, let alone what I might enjoy. I felt like I was holding different puzzle pieces, but I wasn’t sure what image I was building toward.
This summer, I picked up a new piece: my first research project. I’m working in the Soil Ecology Lab with my mentors Antonio Del Vallé and Dr. Meghan Midgley on a project studying brush pile burn scars. Brush pile burning is a common forest management practice used to clear out invasive species like Buckthorn and Honeysuckle. But once the brush burns away, it leaves a bare scar of soil behind that has been overheated, stripped of vegetation, and often ignored in the recovery process. Without intervention, these scars can remain damaged for years, which allows non native species to invade and the loss of microbial communities that keep these ecosystems balanced. My research is exploring different restoration strategies to help these scars heal after burning.
The strategies I’m focusing on are ash removal, reseeding, and organic matter addition. Each of these strategies have their own reasoning: removing the ash to prevent it from leaching into soil and disrupting the pH, adding seeds to help plant regrowth, and layering wood chips or leaf litter to rebuild organic matter. We’re applying these strategies individually and in combination, making us question: what strategies work best to enhance the plant and soil biodiversity in these scars? Each method we try out is like flipping the puzzle pieces around, turning them over, and then looking for connections.
This project has introduced me to both fieldwork and lab work for the first time. In the field I’ve collected soil samples, surveyed plant communities, and learned to identify some different plants. So far, my favorite plant I’ve learned is Spiderwort, a vibrant native flower. As someone who is used to city life in Chicago, being surrounded by forests and tall grasses has been eye-opening (and sometimes itchy– hello, bugs and ticks). I was nervous at first, but I’ve learned that I actually enjoy being out in the field, especially when there’s a bit of shade and it’s not too hot.
In the lab, I began processing the soil samples I collected by sieving out rocks and organic matter, and then weighing them out for different analyses. I’ve learned how to test for phosphorus, inorganic nitrogen, microbial biomass, and pH. I find the precision of lab work surprisingly calming, but it’s also challenging in its own way. Sometimes I would make small mistakes, but what mattered was that I communicated and learned how to fix it. For my first experience in the lab, I don’t think I’ve done so bad, and that makes me happy.
As summer goes on, my plan is to continue gathering data from plant surveys across multiple sites. I’ll be comparing the results from each restoration method to see what encourages the most plant growth and supports healthier soils. I’ll also begin analyzing my soil data, which seems like it might solve this research puzzle, but it might even create more confusion. Who knows! I’m excited to see what results we’ll find.
Every connection I make, whether it’s a new skill, identifying a new plant, or collaborating with my mentors and fellow mentees, it makes me feel like I’m slowly gathering the pieces and putting them all together. So far, the picture I’m seeing is this: I love research, and I love being around people who care deeply about the Earth. Although I still don’t have all the pieces together, like what job I want, or what specific direction I’ll take, I’m so glad I chose a path that I truly enjoy.
I’ve met incredible people who have guided and supported me, and I’m grateful for the opportunity to keep learning and growing as a student and a researcher. For this being my first experience in research, fieldwork, and lab work, I’m proud of the different things I’ve learned about the world and myself.
There’s still more to figure out, but I’m starting to see how it all fits together.
Jamie McManmon
Summer 2025, Binghamton University
New Plant Development Program and Chemical Ecology Lab
“Characterization of Ash Resistance to Emerald Ash Borer”
Up the road from Binghamton University lies a hiking area which once contained a large population of ash trees. While chatting with environmental researchers at my university, I was surprised to hear the number of ash in the area had been decimated due to one pest: the emerald ash borer, or EAB. I was caught off guard at the mention of the insect, as I had just been hired onto this very project. Observing firsthand the devastation EAB caused made this project feel very real to me. As I continued my reading for the experiment I discovered that this invasive species has spread from just Michigan, where it was introduced, to cover the entire eastern United States. Originating from Asia, EAB adults lay their eggs on the bark of American ash trees at which point their larvae hatch and burrow into the tree, killing it through extensive feeding.
The central idea of my project lies with the difference between ash species in the Americas and Asia. Asian ash trees, which evolved alongside the EAB, are capable of killing the larvae that consume their bark before they cause massive damage. A resistance like this means Asian trees nearly always survive infestation, as opposed to American ash, which effectively never do. In order to understand how the resistance happens in Asian ash, my project takes on two angles: chemistry and genetics.
While in the Chemical Ecology lab, I analyze the chemical content of harvested bark in order to observe the presence of certain chemicals theorized to be able to harm EAB larvae. We take samples of each tree and break down their cellular material to extract defense chemicals. These extractions are run through the HPLC (high-performance liquid chromatography) machine, which allows me to find the presence and quantity of certain chemicals so I can understand their role in plant defense.
Meanwhile, working with the New Plant Development lab and their plant genetics specialists is allowing me to analyze the expression of certain genes identified to have a link to EAB resistance and compare their presence in each species. This work involves designing experiments around chosen genes within our plants, then extracting and quantifying genetic material in order to understand how strong its presence is. If I see a high quantity of a gene’s expression within an Asian ash extraction, but not an American ash, I can theorize about that gene’s connection to the tree’s EAB resistance.
With this project, our goal is to strengthen the link between certain factors and EAB resistance, and call others into question. We hope that if we can find significantly more expression of a certain defense chemical or gene in Asian ash compared to American ash, then future studies can focus on that factor as a likely key element to resistance. The information gained through this study can eventually lead to efforts to breed more resistant trees, bringing about a restoration of ash to the American east.
Every day as an REU was different. Some days I’ll be in the lab all day pipetting for extractions, other days I’ll be in the sun harvesting bark samples from the Arboretum’s inventory of young ash trees. When I’m not doing all that hands-on work, I’m coding my statistical analysis or generating graphs of my data. On days where there has been less to do, I would sometimes accompany my mentors to their other responsibilities. This work has brought me out onto the Arboretum grounds to see all sorts of research areas, from neat greenhouses to open fields to offroad dense forests. The work I do is incredibly rewarding, as all of it contributes to a greater understanding of this massive problem. Experience I gain here will help my future efforts to become a researcher in so many ways, and I cannot wait to see where this project leads.
Yoara A. Nadal García
Summer 2025, University of Puerto Rico Rio Piedras
Root Biology Lab
“Tree species variation in belowground resource allocation and uptake”
Ever since I could remember, I have loved nature. Growing up in Puerto Rico, my childhood memories include sitting under big trees, exploring our forests after long roadtrips, and collecting piles of National Geographic magazines. Majoring in environmental science in the University of Puerto Rico-Río Piedras Campus has reaffirmed that I am on the right path, and as an inquisitive student I am constantly searching for opportunities to grow as a scientist. This is what led me to apply to the Morton Arboretum’s REU (Research Experience for Undergraduates) internship, despite utter belief that I wasn’t going to get accepted into the program, and yet, here I am! My advice to people that feel this way is to just go for it. What’s the worst that can happen?
I get to explore my interest in soil by researching root depth and root biomass of different tree species up to 150cm, which is deeper than many previous studies that focus mostly on surficial rooting patterns. Since the decomposition of roots in association with mycorrhizal fungi is a process that adds carbon into the soil, this project helps with carbon estimation belowground and improves predictions for global terrestrial models that work to understand how trees allocate carbon. Most importantly, carbon potentially being stored at deeper depths of soil would mean it’s being stored for a longer period, as its decomposition lowers at deeper increments. This research contributes greatly to climate change mitigation efforts.
Considering my project consists of looking at roots at deeper depths, one could imagine the tube required for the sampling and the equipment to take it out of the ground is heavy, and a bit annoying to deal with every week. As such, we have an ongoing joke that I´m going to get jacked by the end of the summer.
The image below shows Dr. Nicholas Medina and I pulling the metal tube up after it was hammered underground. We do this with two farm jacks and I must confess that I, unbeknownst to many, do enjoy this part a lot.
Then, for another fun part of mine – Research Program Manager Marvin Lo and I work to push the soil out with a stick!
Finally, I section the soil at each measurement and put them in different labeled bags.
The laboratory work consists of separating roots from the soil samples, classifying them, and measuring their weight, as well as saving the soil for future DNA and pH analysis.
Before this experience, I was just beginning to scratch the surface on what environmental science research in laboratories looks like, since my first two undergraduate years were mostly field work focused. Throughout this summer program, not only am I developing my skills in the laboratory, but also acquiring the confidence needed to begin research of my own. Next semester I am planning to work on my undergraduate thesis centered on the vegetation, soil, and geological substrate of an urban forest, and I´m very excited to begin the project with a new set of skills. It’s safe to say that, thus far, it has been a dream come true, especially as someone who always has an endless list of questions that I’m too scared to ask. Plot twist: I’ve been learning to ask them regardless.
Jalen Stovall
Summer 2025, Wartburg College
Arboriculture Lab
“Assessing the effects of pruning on branch structure and resiliency”
Hello, my name is Jalen Stovall. I am an Environmental Science major at Wartburg College in my hometown. I’m interning at The Morton Arboretum this summer.
Before taking this position, I knew very little about the Arboretum, the “Champion of Trees,” and how amazing a place dedicated to learning and plant appreciation could be. I knew even less about conducting and navigating through a research project where I’m the head of the project. My work this summer focuses on reduction pruning, which is basically just the removal of part of a branch back to another smaller branch to allow that one to be the new leader, and how it affects the center of mass and total leaf area of a given branch.
I’d love to say it was smooth sailing since day one, but that would be a complete lie. It was very overwhelming and stressful in the first few weeks. I had minimal tree knowledge, limited pruning background, and wasn’t used to being away from my family. So it was a lot of reading, learning, calls home, and reassurance that I was ready and qualified. The impostor syndrome was very real, and I want people who are reading this to know that it is completely normal and that everyone feels it, even the head honchos with PhDs, but we work through it. Going into the start of week six, I’ve gotten the hang of it for the most part. Living alone away from home for the first time isn’t so hard, and the work is not overly stressful as long as you give it your best and are not afraid to ask for help and ask plenty of questions.
My data collection has been from Autumn Blaze Maples, a hybrid species between the Silver & Red maple. Sample collection has been interesting as it’s not as simple as just cutting off branches and performing our measurements and tests; it’s a whole process of identifying good branches to perform reduction cuts, avoiding previous pruning cuts, and collecting a wide range of branch sizes.
Through the fieldwork process, we came up with the highly coveted two-finger method, which helped us predict where the center of mass of a branch would be before putting it on the pulley, which we used to determine the center of mass of a branch both before and after the reduction cut on the branch. Then other measurements were taken like diameters of branches in various places, distance from the cut to the base, and length of lateral branches and full branch length (using calipers and a reel tape measure).
The most of “fun” part of my project was leaf scanning which was the very labor intensive part of my project, which involved plucking and scanning leaves on the finnickiest machine ever, there was lots of frustration but also lots of good conversation with people in the lab because it was a straightforward repetitive task, and I’ll let you in on a little secret: some movies and shows were definitely played during this process. After scanning them, we placed them in a drying oven to get the dry leaf weight needed for other calculations.
Through the program so far, I’ve learned a lot about tree care and maintenance and how to live on my own for the first time. The Morton support system is amazing as they want to ensure you’re doing okay at work and outside of work, and work-life balance and mental health are very important to them. They want open communication on how you’re doing through the whole program so they can help you succeed and thrive while you’re there. The work culture and everyone wants to see you succeed, and they understand that not everyone has done research before, and they are very patient and helpful throughout the whole learning process.
My overall advice for future REU or people who are thinking about applying is don’t be afraid to ask questions, no matter how straightforward, talk to your coworkers they all have their own unique experiences and path to getting there, and embrace the moment because you’ll only be 20 once, whipping the club cart around the grounds as a “scientist”, so why not enjoy it?
Katerina Winkler
Summer 2025, Weber State University
Herbarium and Systematics Lab
“Leafing Through Specimens: An Exploration into Type Specimens and their Role in Representing Intraspecific Variation”
Do you ever wish you could take a moment, press it between two pieces of paper, and keep it to reference at your convenience? Well, with plants, people have done exactly that for centuries in what are now called herbaria. A herbarium is like a library, though instead of shelves with books organized by the Dewey Decimal System, think of a room filled with filing cabinets containing dried plant specimens, usually organized by family. There are herbaria located all around the world, including at The Morton Arboretum! Each of these dried specimens serve as windows into the past that are vital for plant research of all kinds, including my own.
For every species on Earth, there is a preserved individual that acts as a reference for that species, called a “type specimen”. The type specimen serves to bind a given species’ scientific name, for example, Quercus alba, to a physical representative. For plants, that physical representative is a herbarium specimen.
Within an individual species, there is so much variety. Just take natural human hair and eye color as an example! This raises the question, “How well do these “types” resemble the diversity of the species they should represent?”. Type specimens are often sought after as a reference for a given species. When a species type specimen is a morphological outlier, it can lead to misrepresentation. For instance, if one is describing a new variety of a species, it’s crucial that they compare it directly to the type specimen of the original species. However, if that type specimen isn’t representative of that species’ general morphology, it could lead to taxonomic confusion.
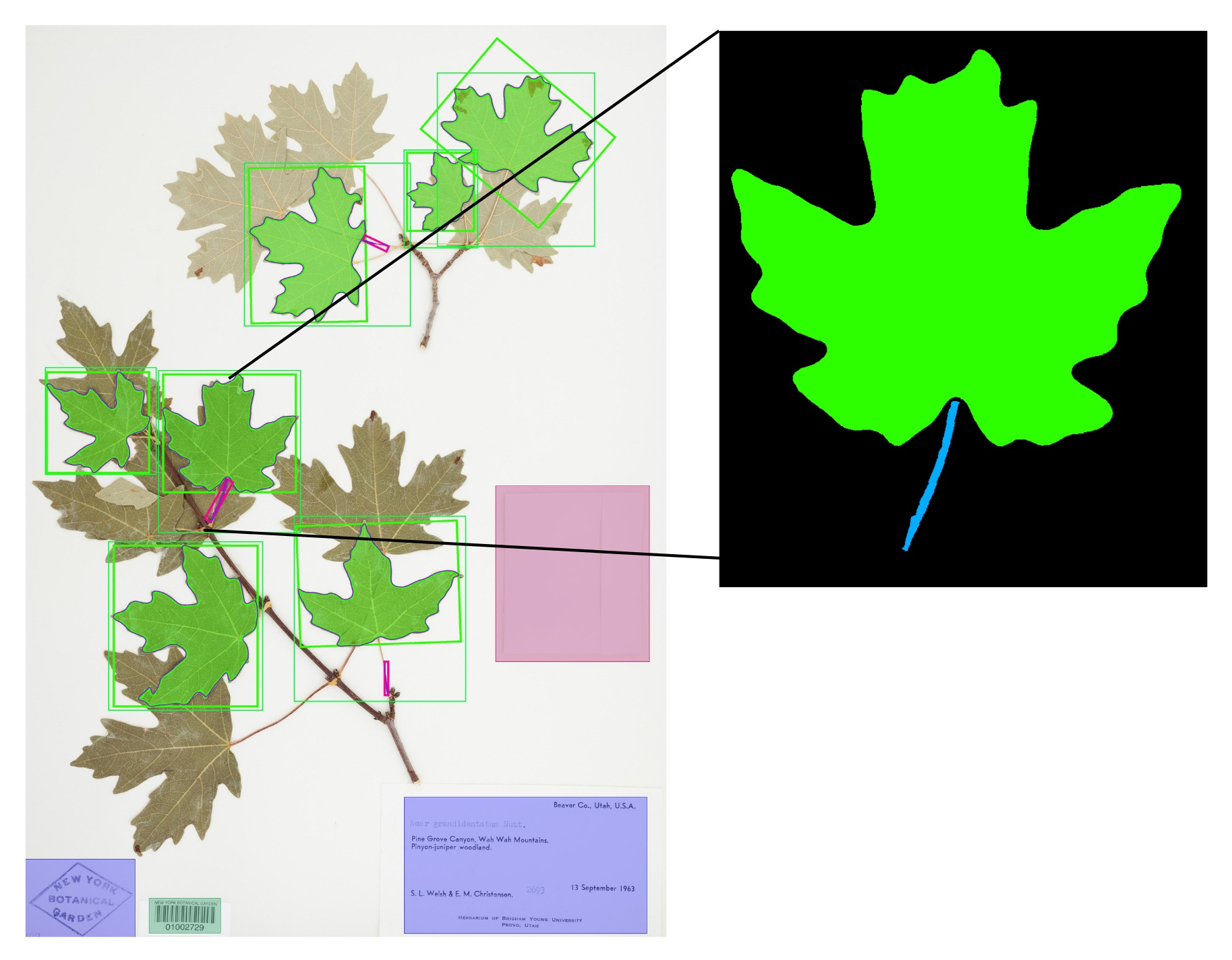
Leaves are important organs for plant identification, and their shape is frequently used as a definitive characteristic. For that reason alone, one can argue that leaf variation should be accurately represented in the type specimen, which is why I have chosen to focus on leaves for my project. Using AI-driven software that performs analysis on the leaves of digital herbarium specimens, I have gathered leaf trait data on a total of sixteen species from six genera.
With this data, I performed an Elliptical Fourier Analysis (EFA) and Principal Component Analysis (PCA) on a total of 14,396 leaf masks. Simply put, the EFA and PCA allow me to summarize the leaf shapes and quantify the amount of leaf variation in each species. Using this mean variation of leaf shape, and comparing it to the type specimen’s leaf shape, I will be able to answer the question, “How well do type specimens represent the leaf variation seen within their respective species?”.
Penny Yost
Summer 2025, Wellesley College
Root Biology Lab
“Characterizing implications of drought induced root shrinkage”
The Morton Arboretum research building sits on the intersection of the east and west sides of the arboretum, at one of the lowest elevations on the grounds. What this means for an REU student who’s decided to explore the arboretum by bike, is that any direction you go with your last 20 minutes of lunch will start uphill. On the other hand, the hard work pays off for a breezy return trip of rolling downhill. If you find a few extra minutes, you can figure-eight back and forth between the east and west sides of the campus: uphill and downhill, past mixed forest and single-species research plots, past birds and rabbits, past curation teams, researchers, and professional tree-climbers. It’s an experience that’s been emblematic of my time helping with research, not just in the healthy balance of ups and downs, but for the chance to be immersed in the world of plant science: stumbling upon new flora and fauna, and seeing a variety of plant science careers in action.
This summer I’m studying how tree roots shrink in drought conditions and how that varies by species. Every Monday, I start the week by collecting root samples of around eight new species to use in the lab the rest of the week. It’s been a lovely chance to explore and see all the corners of the arboretum. In a single morning we collect roots from trees in a prairie to trees in dense, shaded stands. From perfect mulched rings to a bog with a thick understory. Some species are easy to sample. Loose soil and extensive fine-rooting have become far more exciting occurrences in my life than I would ever have guessed. Other species present more challenges. Ponderosa pine, a fragrant pine I grew up loving, struggles in this climate and often has unhealthy, sparse roots at the arboretum. Other species have inconsistent rooting strategies or can only be found surrounded by woody undergrowth (like poison ivy), making identification of target roots difficult (and itchy).
I selected my project for an interest in tree conservation in drought conditions, rather than a specific interest in roots. However, having dived headfirst into the underground world, I cannot think of a better community to spend my summer exploring. Over the last month, I have been introduced to and stumbled across a cast of characters as varied as it is vast. I’ve been charmed by distinct and elegant tulip poplar roots and begrudgingly impressed by the energy efficiency of delicate, spindly oak roots. I’ve begun to learn the root-fungal association types of each species I’ve studied and stumbled across bright purple hyphae. And yet, so little of this diverse and vivid world has been characterized.
Two months ago, I would have believed similarity between the roots of different species could be the only reason we had not thoroughly characterized root variation in the way we had bark or leaves. Yet, I am now able to differentiate the roots of Platanus occidentalis and Platanus x acerifolia. Aboveground, I can only tell these two sycamores apart by reading their tree tags.
The understudied nature of roots makes it not only difficult to identify the target roots of species that have not been well documented, but difficult to predict how my data might correlate to other parts of the hydraulic system of a tree. The more questions I ask about how root-water relations play into this system, the more often the answer is that we don’t have an answer. As difficult as it makes writing a straightforward hypothesis, it’s a lovely and engaging opportunity to collect and analyze data that will help to fill a knowledge gap in our understanding of an integral part of plant drought response.
A few people in my cohort came in early today, and I’ve just gotten back from a short morning ride before work starts, so I’d like to end by expressing how grateful I am to be here. The commonality of all my rides – and time in the lab and field – has been awe at the beauty of the place I have gotten to spend a summer. Because, like my rides around the Arboretum, every part of the experience has been an adventure – often uphill, and frequently sweaty, but always exciting and always surrounded and supported by an amazing community.
2024 Student Blogs
To explore the projects and experiences of the Arboretum’s 2024 cohort of student researchers, please see the 2024 Student Blog Series.
2023 Student Blogs
To explore the projects and experiences of the Arboretum’s 2023 cohort of student researchers, please see the 2023 Student Blog Series.
2022 Student Blogs
To explore the projects and experiences of the Arboretum’s 2022 cohort of student researchers, please see the 2022 Student Blog Series.